
Generally amino acid residues located on the surface of a protein serve as active sites and/or interact with other molecules and ligands. Solvent accessibility plays an important role in the structure and functions of biological macromolecules. One strategy to increase the stability of proteins is to reduce the area of water-accessible hydrophobic surface.
#Examples of hydrophobic amino acids free#
There is a linear relationship between the surface areas of amino acid residues (in a standard state) and the free energy changes associated with the transfer of the amino acids from water to organic solvent. It is now widely accepted that hydrophobicity is a dominant force of protein folding.
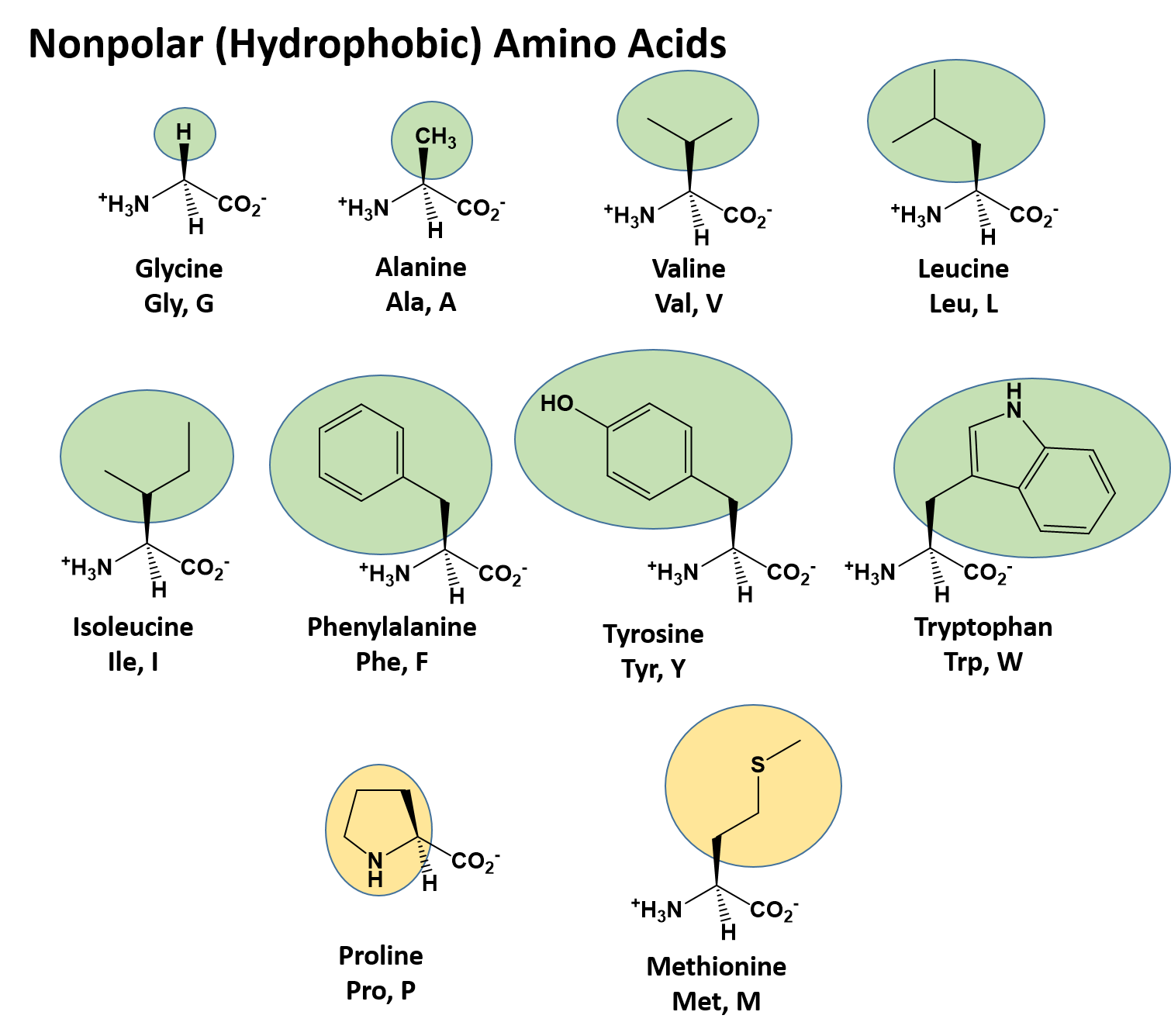
The folding process of polypeptide chain depends on the hydrophobicity of the side chains. In proteins, hydrophobic residues tend to be buried in the interior of the protein away from the solvent and polar side chains are exposed to the solvent. As their name implies, hydrophobic amino acids have essentially nonpolar side chains, for example, valine, leucine, isoleucine, phenylalanine, and methionine fit into this group. It has been hypothesized that hydrophobic interactions play a major role in organizing and stabilizing the architecture of proteins. The hydrophobicity analysis has remained at the central focus for understanding protein folding and stability. This arrangement stabilizes the folded polypeptide backbone, since unfolding it or extending it would expose the hydrophobic side chains to the solvent. The hydrophobic effect is driven by the entropy increase of the solvent water molecules hydrophobic side chains are located predominantly in the interior of a protein. The hydrophobic effect is considered to be the major driving force for the folding of globular proteins. Knowledge of protein stability is crucial for understanding of the basic thermodynamics of the process of folding.

In the absence of a three-dimensional structure, the ability to predict surface accessibility of hydrophobic residues directly from the sequence is of great help in choosing the sites of chemical modification or specific mutations and in the studies of protein stability and molecular interactions.
#Examples of hydrophobic amino acids software#
Solvent accessibility of each protein was determined using NACCESS software and then obtained the homologous sequences to understand how well solvent exposed and buried hydrophobic residues are evolutionarily conserved and assigned the confidence scores to hydrophobic residues to be buried or solvent exposed based on the information obtained from conservation score and knowledge of flanking regions of hydrophobic residues. It is based on the nonredundant data set of 218 monomeric proteins. The present work depends on sequence as well as structural information of the protein and aims to understand nature of hydrophobic residues on the protein surfaces. In general, hydrophobic residues such as Val, Leu, Ile, Phe, and Met tend to be buried in the interior and polar side chains exposed to solvent. The analysis of protein structures provides plenty of information about the factors governing the folding and stability of proteins, the preferred amino acids in the protein environment, the location of the residues in the interior/surface of a protein and so forth.


 0 kommentar(er)
0 kommentar(er)
